
The new FDA annex building and three other room renovations in the main building will enhance the federal agency’s work in Puerto Rico. #NewsismyBusiness

The operation in Puerto Rico includes a team of more than 60 bilingual chemists and microbiologists. #NewsismyBusiness
CEO says the device will create hundreds of manufacturing and other jobs. PapEasy and Ana G. Méndez University will host a cervical cancer symposium today. #NewsismyBusiness

Earlier this month, we updated on the Food and Drug Administration’s efforts to mitigate ongoing IV saline shortages that resulted from, or were worsened by, the devastating impact of Hurricane María in Puerto Rico. We also provided some additional updates related to our continued efforts to help the island fully recover from this disaster.

As we enter the new year and pass the 100-day mark from when Hurricane María made landfall in Puerto Rico, we want to provide an update on the current recovery efforts, the state of existing drug shortages that resulted from the impact of the storm and our continued commitment to help the island fully recover.

While Puerto Rico is making progress in its effort to recover from the devastation left by the hurricanes, it remains a long process and there's a lot of work left to do. At the Food & Drug Administration, we're vigilant about helping address the challenges that remain.

It's been nine weeks since Hurricane Maria made landfall on Puerto Rico and the island continues to struggle to recover from the devastation brought by this storm, as well as Hurricane Irma.
The head of the U.S. Food and Drug Administration, Scott Gottlieb said this week the agency is “doing all it can” to support the immediate needs of Puerto Ricans after Hurricane María’s destruction, as well as ensure supplies of critical drugs produced on the island.

Local multidisciplinary company Pharma Consulting Corp. is marking its 13th anniversary by strengthening its services in Puerto Rico, as well as its footprint in the Central and Latin American regions, company President Charil Pabón said Monday.

The U.S. Customs and Border Protection San Juan Office of Field Operations hosted Wednesday the Automated Commercial Environment (ACE) Symposium to familiarize the local trade community about the transition to a single window for import and export reporting.
The Pharmaceutical Industry Association of Puerto Rico and the federal Food and Drug Administration will host the FDA/PIA 12th Regulatory Meeting Thursday at the Ritz-Carlton San Juan, from 7:30 a.m. to 5 p.m, the trade group announced.

Medical device manufacturer Integra LifeSciences Holdings Corp. was on the receiving end of a warning letter from the U.S. Food & Drug Administration last week, in which it was cited over concerns relating to process validations, corrective and preventative actions, as well as document controls, at its Añasco plant.
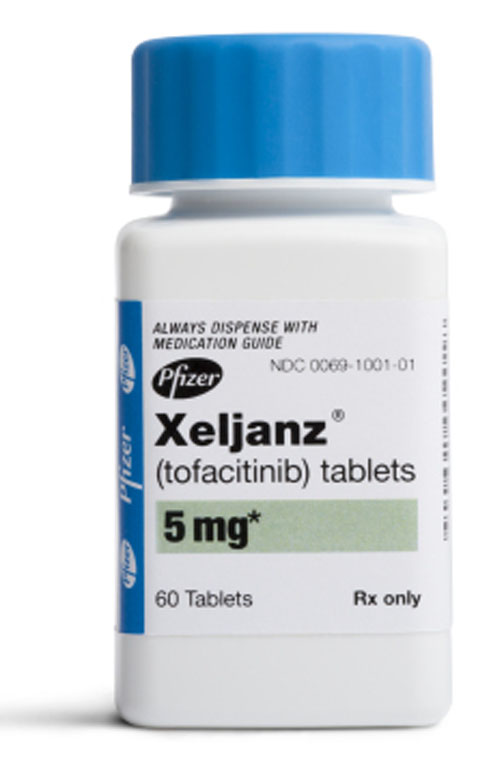
The U.S. Food and Drug Administration recently gave the go-ahead to two new drugs, Xeljanz and Quillivant XR, to treat rheumatoid arthritis and Attention Deficit Hyperactivity Disorder, respectively, the manufacturing companies announced in separate statements.

The Food and Drug Administration has issued a stern warning to pharmaceutical manufacturer Warner Chilcott Company, LLC in Fajardo for not taking sufficient corrective action related to its birth control pill production.

Drugs produced in U.S.-owned and operated pharmaceutical manufacturing plants in Puerto Rico are more likely to have quality problems than those produced by the same firm in a matched plant on the mainland, a study released Tuesday by Ohio State University's Fisher College of Business.




NIMB ON SOCIAL MEDIA